Confocal Laser Scanning Microscopy
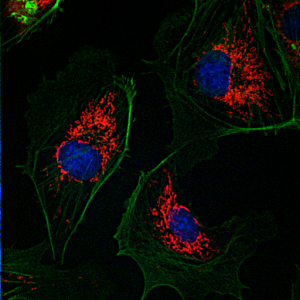 Confocal microscopy is an optical imaging technique used to increase contrast and/or to reconstruct three-dimensional images by using a detection pinhole to eliminate out-of-focus light or flare in specimens that are thicker than the focal plane. This technique has been gaining popularity in the scientific and industrial communities. Typical applications include life sciences and semiconductor inspection.
Confocal microscopy is an optical imaging technique used to increase contrast and/or to reconstruct three-dimensional images by using a detection pinhole to eliminate out-of-focus light or flare in specimens that are thicker than the focal plane. This technique has been gaining popularity in the scientific and industrial communities. Typical applications include life sciences and semiconductor inspection.
DIC (Differential Interference Contrast Microscopy)
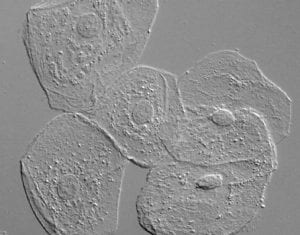 Also known as Nomarski Interference Contrast (NIC) or Nomarski microscopy, is an optical microscopy illumination technique used to enhance the contrast in unstained, transparent samples. DIC works on the principle of interferometry to gain information about the optical density of the sample, to see otherwise invisible features. A relatively complex lighting scheme produces an image with the object appearing black to white on a grey background. This image is similar to that obtained by phase contrast microscopy but without the bright diffraction halo. DIC works by separating a polarized light source into two beams which take slightly different paths through the sample. Where the length of each optical path (i.e. the product of refractive index and geometric path length) differs, the beams interfere when they are recombined. This gives the appearance of a three-dimensional physical relief corresponding to the variation of optical density of the sample, emphasizing lines and edges though not providing a topographically accurate image.
Also known as Nomarski Interference Contrast (NIC) or Nomarski microscopy, is an optical microscopy illumination technique used to enhance the contrast in unstained, transparent samples. DIC works on the principle of interferometry to gain information about the optical density of the sample, to see otherwise invisible features. A relatively complex lighting scheme produces an image with the object appearing black to white on a grey background. This image is similar to that obtained by phase contrast microscopy but without the bright diffraction halo. DIC works by separating a polarized light source into two beams which take slightly different paths through the sample. Where the length of each optical path (i.e. the product of refractive index and geometric path length) differs, the beams interfere when they are recombined. This gives the appearance of a three-dimensional physical relief corresponding to the variation of optical density of the sample, emphasizing lines and edges though not providing a topographically accurate image.
FCS (Fluorescence Correlation Spectroscopy)
A technique used by physicists, chemists, and biologists to experimentally characterize fluorescent molecules and their dynamics. Using confocal or two photon microscopy, light is focused on a sample and the measured fluorescence intensity fluctuations (due to diffusion, chemical reactions, aggregation, etc.) are analyzed using the temporal autocorrelation. FCS is the fluorescent counterpart to dynamic light scattering, which uses coherent light scattering, instead of (incoherent) fluorescence. FCS obtains quantitative information such as diffusion coefficients, hydrodynamic radii, average concentrations, and kinetic chemical reaction rates. Because fluorescent markers come in a variety of colors and can be specific to a particular molecule, molecules can be studied individually and in rapid succession in composite solutions. The advent of engineered cells with genetically tagged proteins (like green fluorescent protein) has made FCS a common tool for studying living cells.
FLIP (Fluorescence-Loss-In-Photobleaching)
Related to FRAP. Typically a specific area of a cell membrane previously labelled with a floating fluorescent dye is bleached with a confocal laser scanning microscope and the loss or recovery of fluorescence over time is recorded to determine the lateral membrane fluidity. In addition FLIP is useful in verifying the continuity of membranous organelles. By continuous bleaching of a small circumscribed organelle area the fluorescence from the non-illuminated part slowly vanishes, if unbleached fluorophores can laterally diffuse into the illuminated spot until all fluorescent molecules are depleted.
FRAP (Fluorescence Recovery After Photobleaching)
An optical technique capable of quantifying the two dimensional lateral diffusion of a molecularly thin film containing fluorescently labeled probes. This technique provides great utility in biological studies of cell membrane diffusion and protein binding. In addition, surface deposition of a fluorescing phospholipid bilayer (or monolayer) allows the characterization of hydrophilic (or hydrophobic) surfaces in terms of surface structure and free energy. Similar, though less well known, techniques have been developed to investigate the 3-dimensional diffusion and binding of molecules inside the cell; they are also referred to as FRAP.
FRET (Fluorescence/ Forster Resonance Energy Transfer)
An optical technique that involves energy transfer between two fluorophores. A donor fluorophore in its excited state can transfer energy by a nonradiative, long-range dipole-dipole coupling mechanism to an acceptor fluorophore in close proximity (typically <10nm). When both molecules are fluorescent, the term “fluorescence resonance energy transfer” is often used, although the energy is not actually transferred by fluorescence.
Live-cell Imaging
Live-cell imaging techniques provide critical insight into the fundamental nature of cell and tissue function, especially due to the rapid advances that are currently being witnessed in fluorescent protein and synthetic fluorophore technology. Tight control of the environment is one of the most critical factors in successful live-cell imaging experiments. In particular, the conditions under which cells are maintained on the microscope stage, although widely variable in many requirements depending upon the organism, often dictate the success or failure of an experiment. Aspects of the environment that are readily manipulated include the physical parameters of the chamber in which the cells are grown and imaged, the localized degree of temperature control, atmospheric conditions (gas mixture and humidity), nutritional supplements, growth medium buffering (pH), and osmolarity of the culture medium.
Phase Contrast Microscopy
An optical microscopy illumination technique in which small phase shifts in the light passing through a transparent specimen are converted into amplitude or contrast changes in the image. A phase contrast microscope does not require staining to view the slide.
Widefield Fluorescence Microscopy
This method of microscopy that is widely used in life sciences. The excitatory light is passed from above (or, for inverted microscopes, from below), through the objective and then onto the specimen instead of passing it first through the specimen. (In the latter case the transmitted exitatory light reaches the objective together with light emitted from the specimen). The fluorescence in the specimen gives rise to emitted light which is focused to the detector by the same objective that is used for the excitation. A filter between the objective and the detector filters out the excitation light from fluorescent light. Since most of the excitatory light is transmitted through the specimen, only reflected excitatory light reaches the objective together with the emitted light and this method therefore gives an improved signal to noise ratio. A common use in biology is to apply fluorescent stains to the specimen to provide an estimated count.
Image Processing and Analysis
An essential component of imaging is processing the images and analyzing the data contained within the images. FIJI/ImageJ is an open source software that can be downloaded from http://fiji.sc for free. More information and Tutorials are available on our Image Analysis page as well as at ImageJ.net.